OLM Optical Light micrographs of Tasmanian tiger hair
Abstract
One hundred and seventeen micrographs utilising brightfield light microscopy are presented, to illustrate key diagnostic features of Tasmanian tiger hair (Thylacinus cynocephalus, also called thylacine). All three hair types are copiously represented. Surface scale patterns are visible traversing fibres and as scales raised one above the next, at fibre edges. Medullary structure and scale patterns are apparent. The general morphology of a thinner root, very thin tip and wider mid section is demonstrated. Changes in fibre colouration are observed and some atypical features are described.
Nomenclature for morphological features of hair fibres by Deedrick and Koch is summarised and used throughout. Nomenclature used by Lyne & McMahon and by Taylor is reviewed and conflicting. A consistent nomenclature after Taylor's key is encouraged. A summary table of micrographs is given outlining the fibre types visible within each micrograph, the portion of the fibre visible and form of the medullary structure, medullary cell pattern and scale pattern, if discernible.
The key purpose of this study is to provide examples of thylacine hair utilising light microscopy as a basis to help identify unknown hair samples collected in the field. On the basis of Taylor's key, a final confirmation of a prospective thylacine hair utilising the images presented here, will require verification of the cross-sectional shape of the prospective hair. However this study illustrates that the other checks in Taylor's key may be completed first, using whole mounts of the hair fibres under brightfield light microscopy, which requires the simplest possible slide preparation. The present study extends the scope of earlier research on thylacine hair by examining under and over hairs (in addition to guard hairs) in detail.
Overview
Multiple series of micrographs of Tasmanian tiger hair (Thylacinus cynocephalus, also called "thylacine") were produced in 2017 by Rehberg (author), in collaboration with the Commonweath Scientific and Industrial Research Organisation (CSIRO). These included scanning electron microscopy (SEM) with and without metal coating, and confocal laser scanning microscopy (CLSM).
The sequence of micrographs presented here has been produced using brightfield optical light microscopy and complements the earlier micrographs. These images represent the end goal of a crowd-funding project designed to deliver high resolution, colour images, as reference material for researchers utilising relatively inexpensive and accessible optical light microscopy techniques.
Technique
The Tasmanian tiger's fur coat includes three distinct types of hair (Lyne & McMahon, 1950; Taylor, 1985) described as "under", "guard" and "over" hairs. The hair fibres used in this research were drawn from the same sample as described in Rehberg 2017 dated between approximately 1850 and 1890. The individual under and guard hair fibres were distinct from those used during earlier work. However due to scarcity of over hair fibres in the sample, the over hair fibre used in this research is one used during the earlier metal-coated SEM work and has been coated with a thin layer (~2 nanometres) of platinum/palladium.
The following five slides were prepared by dry mounting fibres onto a glass slide using tweezers, then covering the samples with cover slips:
- Slide 1: Two under hair fibres
- Slide 2: Two guard hair fibres
- Slide 3: Two guard hair fibres
- Slide 4: Four guard hair fibres (only one fibre imaged)
- Slide 5: One over hair fibre
Slides were mounted onto an Omano OM88-T Trinocular Compound Microscope. An LM Digital SLR Widefield Adapter was used to attach a Nikon D800E digital SLR camera to the microscope. The camera was set to Program (automatic exposure) mode utilising "Live View" preview on the rear screen and a time delay was used between shutter operation and image capture in order to minimise camera movement. Focusing was achieved by visual inspection of the camera's screen. RAW data and a low resolution thumbnail were captured with each exposure. The camera was also used in Live View video mode, to capture video sequences.
Photographs were taken with either a 4x, 10x or 40x objective in place. 10x eyepieces were in place for the binocular view but the camera port did not contain any eyepiece. Nevertheless, the (rectangular) view on the camera screen approximated the enlargement seen through the (circular) view of the eye pieces and thus enlargement sizes at time of image capture are assumed to approximate 40x, 100x and 400x respectively. Display size on your screen or printout will vary.
Scale bars were produced by photographing a Carl Zeiss Jena 1/0,01 scale slide under the same magnifications. Scale bars follow the same format used in the SEM results: a series of 11 points is displayed and the distance between end points is given beneath them (being 1,000, 400 or 100 micrometres, respectively). The distance between two adjacent points is one tenth of this value.
RAW image data were imported into Adobe Lightroom 4.4. This produced images of 7,360 pixels along the longest edge. Images were rotated 90 degrees from portrait to landscape orientation, exported to JPEG format, downsized for web distribution and watermarked.
Images
Images were captured for all three hair types: under hair; guard hair; over hair.
The primary goal in the production of the light micrographs is to provide a reference against which researchers might compare unidentified hair fibres collected "in the field" in the search for evidence of the thylacine. The earlier SEM and CLSM work gave unprecedented detail to our understanding of thylacine hair surface morphology and scale patterns. Light microscopy, in contrast, produces lower resolution images with fewer visible details. It also provides insight into the appearance of the internal structures of the hair as light passes through the fibre. While access to SEM and CLSM services is likely to be prohibitively expensive or inaccessible to many researchers, light microscopy is relatively affordable and accessible. In preparing these light micrographs attention has been given to attempting to match the surface scale patterns revealed in detail by the SEM and CLSM work with their appearance under the more restrictive conditions of light microscopy.
One property of high magnification using light microscopy is that the focal depth of field is much shallower than the thickness of a hair fibre. That is, even though a given fibre may be only 50 micrometres in diameter, under high magnification, the light microscope may focus on approximately only 5 micrometres of depth at a time. Further, as focus is shifted from the uppermost surface of the fibre downwards, the light microscope begins to reveal the internal structure of the fibre. Hence at one focal point, surface detail may be seen but with a tiny focal adjustment, surface detail disappears from view and internal structure is seen. Interpreting the internal and external morphology of the fibre tends to rely on a process of constantly adjusting focus up and down through the fibre whilst watching in real time. An individual micrograph can only convey the view at a single point during this process. For this reason, where interesting features have been noted, several micrographs have been produced at the same point along the fibre but at different focal depths.
File names are given for each image as four digit numbers. These may be used to uniquely identify individual micrographs.
All images are copyright.
Due to the volume of images and their file sizes, the micrographs have been published on three separate pages.
Under hair micrographs
Go to Under Hair Results (28 images totaling 16MB)
Guard hair micrographs
Go to Guard Hair Results (52 images totaling 29MB)
Over hair micrographs
Go to Over Hair Results (37 images totaling 20MB)
Results
A total of 117 whole-mount micrographs of Tasmanian tiger hair have been produced. These consist of 28 under hair images, 52 guard hair images and 37 over hair images. Under hair and guard hair fibres were each imaged at 100x and 400x magnifications. The over hair fibre was imaged at 40x, 100x and 400x magnifications. Many of the features described by Lyne & McMahon, and also by Taylor, are visible under light microscopy.
A summary of the physiological features of hair fibres that are visible under light microscopy is given below.
A number of physiological features are visible with Tasmanian tiger hair under light microscopy. These are summarised here and then a discussion of those features visible in thylacine hair is given.
Nomenclature of physiological features
Deedrick and Koch present nomenclature for scale patterns, medullary structure and medullary cell patterns (2004). Their descriptions are summarised here.
Scale patterns
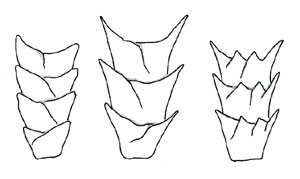
"There are three basic scale structures that make up the cuticle—coronal (crown-like), spinous (petal-like), and imbricate (flattened)" (Deedrick and Koch, 2004).
Table of scale patterns, after Deedrick and Koch (2004).
| Feature | Illustration | Example micrograph (not thylacine) | Description |
|---|---|---|---|
| Coronal scales | "The coronal, or crown-like scale pattern, is found in hairs of very fine diameter and resemble a stack of paper cups. Coronal scales are commonly found in the hairs of small rodents and bats but rarely in human hairs." "Photomicrograph of Free-Tailed Bat Hair" | ||
| Spinuous scales | "Spinous or petal-like scales are triangular in shape and protrude from the hair shaft. They are found at the proximal region of mink hairs and on the fur hairs of seals, cats, and some other animals. They are never found in human hairs." "Photomicrograph of Proximal-Scale Pattern (Mink)" | ||
| Imbricate scales | "The imbricate or flattened-scale type consists of overlapping scales with narrow margins. They are commonly found in human hairs and many animal hairs." "Photomicrograph of Distal-Scale Pattern (Mink)" |
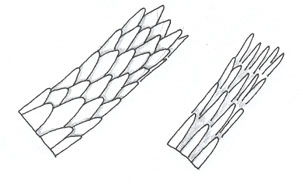
Medullary structure patterns
Deedrick and Koch describe the medulla as "a central core of cells that may be present in the hair. If it is filled with air, it appears as a black or opaque structure under transmitted light, or as a white structure under reflected light. If it is filled with mounting medium or some other clear substance, the structure appears clear or translucent in transmitted light, or nearly invisible in reflected light. In human hairs, the medulla is generally amorphous in appearance, whereas in animal hairs, its structure is frequently very regular and well defined" (2004).
Table of medullary patterns, after Deedrick and Koch (2004).
| Feature | Illustration |
|---|---|
Fragmentary medulla also known as Trace medulla | |
Discontinuous medulla also known as Broken medulla | |
| Continuous medulla |
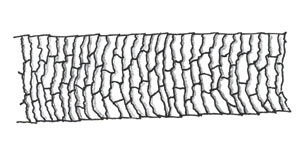
Medullary cell patterns
Deedrick and Koch also describe the appearance of the cells within the medulla.
Table of medullary cell patterns, after Deedrick and Koch (2004).
| Feature | Micrograph |
|---|---|
| Uniserial-Ladder Medulla | |
| Multiserial-Ladder Medulla | |
| Cellular or Vacuolated Medulla | |
| Lattice Medulla |
Physiological Features visible in thylacine hair under light microscopy
The table below lists the physiological features - using the terminology of Deedrick and Koch - that are visible in the micrographs produced using whole mounts of thylacine hair.
Key to table of results
| Column | Notes |
|---|---|
| File | Unique identifier for the image. |
| Hair Type | Type of hair fibre visible in the image: under; guard; over. |
| Fibre | Unique identifier for the individual fibre visible within this image. Eight unique fibres have been imaged (Under, fibres 1 - 2; Guard, fibres 3 - 7; Over, fibre 8). |
| Region | The region of the fibre visible in the image: Mid Section; Proximal End (if the end is definitively identifiable as toward the root, even if terminated prior to the root); Distal End (if the end is definitively identifiable as the terminus of the fibre). |
| Ending | Comments on how the fibre terminates at this end: Natural; Cut; Split; Broken; Rounded; Tapered. |
| Captured at | The magnification at which the image was captured: 40x; 100x; 400x. |
| Remaining columns | 3 columns representing scale types; 4 columns representing medullary structure pattern; 3 columns representing medullary cell pattern. Within these columns:
|
Table of results
Table of physiological features visible in the published micrographs
Note: Image 2949 is listed twice in the table below, with one line for each of the two fibres visible in that image.
Fibre 5 (a guard hair) is imaged for its entire length at maximum magnification.
| Scale Pattern | Medullary Structure Pattern | Medullary Cell Pattern | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File | Hair Type | Fibre | Region | Ending | Cap'd at | Coronal | Spinuous | Imbricate | Absent | Fragment -ary | Broken | Contin -uous | Uniserial Ladder | Multiserial Ladder | Vacuol -ated | Lattice |
| 2948 | Under | 1 | Proximal End | 100x | yes | yes | yes | yes | yes | |||||||
| 2949 | Under | 1 | Distal End | Natural, split | 100x | |||||||||||
| 2949 | Under | 2 | Distal End | Cut | 100x | yes | yes | |||||||||
| 2950 | Under | 2 | Distal End | 100x | yes | yes | yes | |||||||||
| 2951 | Under | 2 | Mid | 100x | yes | yes | ||||||||||
| 2952 | Under | 2 | Mid | 100x | yes | yes | ||||||||||
| 2953 | Under | 2 | Mid | 100x | yes | yes | ||||||||||
| 2954 | Under | 2 | Mid | 100x | yes | yes | ||||||||||
| 2955 | Under | 2 | Mid | 100x | yes | yes | ||||||||||
| 2956 | Under | 2 | Mid | 100x | yes | yes | ||||||||||
| 2957 | Under | 2 | Mid | 400x | yes | yes | ||||||||||
| 2959 | Under | 2 | Proximal End | Cut | 400x | yes | yes | |||||||||
| 2960 | Under | 2 | Mid | 400x | yes | |||||||||||
| 2961 | Under | 2 | Mid | 400x | yes | yes | ||||||||||
| 2963 | Under | 2 | Proximal End | Cut | 400x | yes | yes | |||||||||
| 2964 | Under | 2 | Proximal End | Cut | 400x | yes | yes | |||||||||
| 2965 | Under | 1 | Mid | 400x | yes | yes | ||||||||||
| 2966 | Under | 1 | Proximal End | 400x | yes | yes | ||||||||||
| 2967 | Under | 1 | Mid | 400x | yes | yes | ||||||||||
| 2968 | Under | 1 | Mid | 400x | yes | yes | ||||||||||
| 2970 | Under | 1 | Mid | 400x | yes | yes | yes | yes | ||||||||
| 2971 | Under | 1 | Mid | 400x | yes | yes | yes | |||||||||
| 2972 | Under | 1 | Mid | 400x | yes | yes | ||||||||||
| 2973 | Under | 1 | Mid | 400x | yes | yes | ||||||||||
| 2974 | Under | 1 | Mid | 400x | yes | yes | yes | |||||||||
| 2975 | Under | 1 | Mid | 400x | yes | yes | ||||||||||
| 2976 | Under | 1 | Mid | 400x | yes | yes | ||||||||||
| 2977 | Under | 1 | Mid | 400x | yes | yes | ||||||||||
| 2978 | Under | 1 | Distal End | Split | 400x | yes | yes | |||||||||
| 2980 | Guard | 3 | Proximal End | 100x | yes | yes | ||||||||||
| 2981 | Guard | 3 | Mid | 100x | yes | yes | yes | |||||||||
| 2982 | Guard | 3 | Mid | 100x | yes | yes | yes | |||||||||
| 2983 | Guard | 3 | Mid | 100x | yes | yes | yes | |||||||||
| 2984 | Guard | 3 | Mid | 100x | yes | yes | ||||||||||
| 2986 | Guard | 4 | Distal End | 400x | yes | |||||||||||
| 2987 | Guard | 4 | Mid | 400x | ? | |||||||||||
| 2988 | Guard | 4 | Mid | 400x | yes | yes | ||||||||||
| 2989 | Guard | 4 | Mid | 100x | yes | yes | yes | |||||||||
| 2992 | Guard | 4 | Proximal End | Broken | 400x | yes | ||||||||||
| 2993 | Guard | 4 | Proximal End | Broken | 400x | yes | yes | yes | ||||||||
| 2994 | Guard | 4 | Proximal End | Broken | 400x | yes | yes | |||||||||
| 2995 | Guard | 4 | Proximal End | Broken | 400x | yes | yes | |||||||||
| 2997 | Guard | 5 | Proximal End | Broken | 100x | yes | yes | yes | yes | |||||||
| 2999 | Guard | 5 | Proximal End | Broken | 400x | yes | ||||||||||
| 3000 | Guard | 5 | Mid | 400x | yes | yes | yes | yes | yes | |||||||
| 3001 | Guard | 5 | Mid | 400x | yes | yes | yes | yes | yes | |||||||
| 3002 | Guard | 5 | Mid | 400x | yes | yes | yes | yes | ||||||||
| 3003 | Guard | 5 | Mid | 400x | yes | yes | yes | |||||||||
| 3004 | Guard | 5 | Mid | 400x | yes | yes | yes | |||||||||
| 3005 | Guard | 5 | Mid | 400x | yes | yes | yes | |||||||||
| 3006 | Guard | 5 | Mid | 400x | yes | yes | yes | |||||||||
| 3007 | Guard | 5 | Mid | 400x | yes | yes | ||||||||||
| 3008 | Guard | 5 | Mid | 400x | yes | yes | ||||||||||
| 3009 | Guard | 5 | Mid | 400x | ||||||||||||
| 3010 | Guard | 5 | Distal End | Rounded | 400x | |||||||||||
| 3011 | Guard | 6 | Proximal End | Broken | 100x | yes | yes | |||||||||
| 3012 | Guard | 6 | Mid | 100x | yes | yes | ||||||||||
| 3013 | Guard | 6 | Distal End | 100x | ||||||||||||
| 3014 | Guard | 6 | Distal End | 100x | yes | |||||||||||
| 3015 | Guard | 6 | Mid | 400x | yes | yes | yes | |||||||||
| 3016 | Guard | 6 | Mid | 400x | yes | yes | ||||||||||
| 3017 | Guard | 6 | Mid | 400x | yes | yes | yes | |||||||||
| 3018 | Guard | 6 | Mid | 400x | yes | yes | yes | |||||||||
| 3019 | Guard | 6 | Proximal End | Broken | 400x | yes | yes | yes | ||||||||
| 3020 | Guard | 6 | Proximal End | Broken | 400x | yes | yes | |||||||||
| 3022 | Guard | 7 | Proximal End | Broken | 400x | yes | yes | yes | yes | |||||||
| 3023 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3024 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3025 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3026 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3027 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3028 | Guard | 7 | Mid | 400x | yes | |||||||||||
| 3029 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3030 | Guard | 7 | Mid | 400x | yes | yes | yes | |||||||||
| 3031 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3032 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3033 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3035 | Guard | 7 | Distal End | Tapered | 400x | yes | ||||||||||
| 3036 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3037 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 3038 | Guard | 7 | Mid | 400x | yes | yes | ||||||||||
| 4013 | Over | 8 | Proximal End | Broken | 40x | yes | yes | |||||||||
| 4014 | Over | 8 | Mid | 40x | yes | yes | ||||||||||
| 4015 | Over | 8 | Mid | 40x | yes | yes | ||||||||||
| 4016 | Over | 8 | Mid | 40x | yes | yes | ||||||||||
| 4017 | Over | 8 | Mid | 40x | yes | yes | ||||||||||
| 4018 | Over | 8 | Mid | 40x | yes | yes | ||||||||||
| 4019 | Over | 8 | Mid | 40x | yes | yes | ||||||||||
| 4020 | Over | 8 | Distal End | 40x | yes | yes | yes | |||||||||
| 4021 | Over | 8 | Distal End | 40x | yes | yes | yes | |||||||||
| 4022 | Over | 8 | Distal End | 40x | yes | yes | yes | |||||||||
| 4023 | Over | 8 | Distal End | 100x | yes | yes | yes | |||||||||
| 4024 | Over | 8 | Mid | 100x | ||||||||||||
| 4025 | Over | 8 | Mid | 100x | ||||||||||||
| 4026 | Over | 8 | Mid | 100x | yes | yes | ||||||||||
| 4027 | Over | 8 | Mid | 100x | yes | yes | ||||||||||
| 4028 | Over | 8 | Mid | 100x | yes | yes | ||||||||||
| 4029 | Over | 8 | Mid | 100x | yes | yes | ||||||||||
| 4030 | Over | 8 | Mid | 100x | yes | yes | ||||||||||
| 4031 | Over | 8 | Mid | 100x | yes | yes | ||||||||||
| 4032 | Over | 8 | Mid | 100x | yes | yes | ||||||||||
| 4033 | Over | 8 | Mid | 100x | yes | yes | ||||||||||
| 4034 | Over | 8 | Mid | 100x | ? | ? | yes | yes | ||||||||
| 4035 | Over | 8 | Mid | 100x | yes | yes | ||||||||||
| 4036 | Over | 8 | Mid | 100x | yes | yes | yes | |||||||||
| 4038 | Over | 8 | Mid | 100x | yes | yes | yes | |||||||||
| 4039 | Over | 8 | Mid | 100x | yes | yes | yes | |||||||||
| 4040 | Over | 8 | Proximal End | Broken | 100x | yes | yes | yes | ? | ? | ||||||
| 4041 | Over | 8 | Proximal End | Broken | 100x | yes | ? | ? | ||||||||
| 4042 | Over | 8 | Proximal End | Broken | 400x | yes | yes | ? | ? | |||||||
| 4043 | Over | 8 | Proximal End | Broken | 400x | yes | ? | ? | ||||||||
| 4044 | Over | 8 | Proximal End | Broken | 400x | yes | ? | ? | ||||||||
| 4045 | Over | 8 | Proximal End | Broken | 400x | yes | ? | ? | ||||||||
| 4046 | Over | 8 | Mid | 100x | yes | yes | ? | ? | ||||||||
| 4047 | Over | 8 | Distal End | Tapered | 400x | yes | ||||||||||
| 4048 | Over | 8 | Mid | 400x | yes | |||||||||||
| 4049 | Over | 8 | Mid | 400x | yes | |||||||||||
| 4050 | Over | 8 | Mid | 400x | yes | |||||||||||
Discussion
Usefulness of the light micrographs in identifying thylacine hair
Speaking of Tasmanian mammals broadly, Lyne & McMahon note "the observations on the cuticular scale patterns alone, without considering other elements of the hair shaft, are of only limited value in comparative study of marsupial hairs" (p 71). They note "marked changes in scale form, which are usually found along a single hair, are considered to be of an inherent and genetic character and are not caused by varying amounts of attrition or by dehydration of the hair shaft" and that the cross-sectional shape is a "useful character in the identification of Tasmanian monotreme and marsupial hair".
Taylor expands on Lyne & McMahon's earlier work to prepare a key for identifying Tasmanian mammal hair. The "initial separation of groups is based on the cross-sectional appearance of primary guard hairs [at their widest point]" (p 69). Thylacinus cynocephalus falls into Group 3, having primary guard hairs that are "predominantly oblong in cross-section".
In both cases the authors contend that cross sectional shape is integral to any mystery hair identification. Cross sectional analysis has not been part of the present study. As such, a firm identification for thylacine hair can only be made once a candidate hair is verified to have a cross-sectional shape as described by Taylor. Reversing the logical steps in Taylor's key - so that the cross-sectional analysis is the last step - has several advantages: whole mounts are simpler and quicker to prepare than cross sectional mounts and are non-destructive of the hair fibre. The value of the images in this study, then, is as an initial screening process for either eliminating or verifying the possibility that a candidate hair may come from the thylacine. Those fibres conforming with thylacine scale and medulla morphology may then undergo cross-sectional analysis as a final verification of the identification of the hair - as being thylacine or not.
Taylor includes some micrographs revealing medullary cell pattern (Plate 6, figures A to D, pp 76,7). The present study includes ample image of all hair types revealing both scale pattern under light microscopy (ie. without the need for preparing any casts) as well as medullary structure and medullary cell patterns.
A limitations in Taylor's key is that it applies only to "primary guard hairs ... found on the main pelage" and that hair "from the extremities can sometimes differ from that on the main pelage" (p71). Prospective thylacine guard hairs may be readily distinguished from the other hair types by their gross morphology, being approximately 16mm in length, straight for approximately 40% of the length and then wavy (Rehberg, 2017 - SEM). If an unknown candidate hair sample better matches the description of the longer over hair or short, wavy under hair, then the images in this study may be of assistance in establishing whether the candidate hair is consistent with a documented thylacine hair but the identification cannot be taken as conclusive.
It is also important to note that Lyne & McMahon's terminology differs from Taylor's. Where Lyne & McMahon reference "protective hairs" (p73) this is understood to mean "guard hairs" as described by Taylor. Where Lyne & McMahon reference "fur hairs" (p73), this is understood to mean "under hairs" as described by Taylor. Lyne & McMahon supply the approximate length of guard hairs as 16mm (as quoted above) whereas the whole sample photography by Taylor, given with a scale, suggests guard hairs may be 20 - 23mm (measured in a straight line, from end point to end point) and certainly much more than 2mm longer than under hairs. In this study the guard hairs were, on visual examination, clearly more than 2mm longer than guard hairs and consistent with Taylor's relative lengths.
Proposing consistent terminology for thylacine hair types
Overall both Taylor and Lyne & McMahon have used varying terminology in describing hair types, even when specific terms are defined. For example, the present study follows the terminology adopted during the scanning electron microscopy work (Rehberg, 2017 - SEM) which was based on Taylor's whole sample photographs (ie. over hair; guard hair; under hair) yet within the same article Taylor refers to "primary guard hairs" (p69, with no explanation of the term 'primary') and "primary hair" (p 76). Lyne & McMahon state that "In general, all [mammalian] hair can be divided into two types; the protective, or guard hair, and the underfur or fur hair. The protective hair is usually long, coarse and straight" (ie better matching Taylor's over hair photograph, p 76 but in contrast with the same term "protective hair" on Lyne & McMahon's page 73, which is described as 16mm in length) "whereas the fur hair is usually shorter, fine and woolly" (by which this author understands "woolly" to mean "wavy"). "... These two types of hair may be quite obvious but sometimes they are difficult to detect, and occasionally there is no definite line of demarcation between them" (p 75). They go on to note that "in all the Tasmanian representatives of [Family Dasyuridae], except Thylacinus, the coat is composed of two distinct types of hair" (p 77). Illustrations of thylacine scale patterns are labelled variously as "wavy hair" and "straight hair". It is proposed that future studies referring to thylacine hair adopt Taylor's descriptions of over, guard and under hair and that terms "protective", "underfur", "fur", "wavy", "woolly", "straight" and "primary" be avoided as sole descriptors for any of the three hair types.
Notable features observed under light microscopy
A summary of various features notable for thylacine hairs under light micrscopy is given below. These are discussed in detail alongside the relevant micrographs.
- The root end of under hairs lacks a medulla for some notable distance, and is relatively transparent.
- The root end of guard hairs also lacks a medulla for a comparable distance.
- The distal end of guard hairs lacks a medulla for a considerably longer distance than at the root end of either guard or under hairs.
- The root end of under hairs shows an imbricate scale pattern before very quickly exhibiting a spinuous scale pattern.
- The visual appearance of the medulla near the root end of guard hairs is definable and readily recognisable: as the medulla forms, it is rapidly tapering, perhaps with a break, and quickly spans almost 100% of the width of the fibre. The fibre's overall width increases in conjunction with the widening medulla.
- Four of the five guard hairs were broken at the point where the medulla forms near the root.
- The distal end of guard hair has dark banding (that is, a striped pattern) against dark brown, where the medulla is lacking. In some guard hairs this banding does not extend to the very tip of the fibre, which is a straw colour. In other guard hairs the banding extends to the tip, which is dark brown and in these cases it seems likely the very tip (ie. unbanded straw coloured tip) has been broken away.
- The distal end of the single over hair sample also has dark banding against dark brown where the medulla is lacking and this banding does not extend to the very tip, which is straw coloured. That is, the over hair appears similar to some of the (presumably more intact) guard hairs at the distal end. With this single over hair being prematurely broken at its proximal end, the defining characteristics separating it from the guard hairs is its greater (implied) length, lack of waviness, thicker diameter and the fact that the intact end is clearly a distal end which, in guard hairs, is wavy, but in this hair, is straight.
- Colourations visible amongst the hair types, at various points, include transparent, straw-coloured (that is, light brown, or sandy brown) and dark brown (ie chocolate brown).
- The medulla appears relatively thinner in the over hair compared with guard hairs. Given the over hair is thicker, this suggests that medullary cells are of a relatively fixed diameter between those two hair types and that it is the thickness of the cortex that varies between guard and over hair.
- The over hair exhibits atypical features at its break point, these being an apparent split along the fibre's length, on one side only, and some vacuolation of the medullary cells for some distance.
- By varying focal depth, the scale pattern may be observed on whole mounts at the higher magnifications. Images captured at 400x magnification clearly show imbricate patterns when focus is on the fibre surface, and the overlapping of scales along the edge of fibres when focus is central to the fibre. Images captured at 100x magnification are suggestive of the expected scale patterns, both on the surface and at the fibre edges.
- The spinuous scale patterns of the under hair are readily visible even at 100x magnification near the root of the fibre.
As a side note, by examining the gross morphology of guard hairs, it is apparent that the straight end of each fibre is notably thicker than the wavy end (even to the naked eye). By confirming the lay of the scales in this study it is established that the thick (ie. straight) end of the guard hair is the proximal end; the thin, wavy end is the distal end.
Possible relationship between medulla, scale pattern and fibre width
Lyne & McMahon note that "the majority of hair shafts show some variation in diameter throughout their length. The base is usually of less diameter than the mid-region. In most of the Tasmanian marsupials the protective hairs are widest distal of the mid region and the tip usually tapers off to practically nothing" (p 76).
This pattern of a relatively thin proximal end, wider mid region, and very thin tip (after some length of tapering) was clearly observable in the under and guard hairs in this study. The root end of the over hair used in this study is missing but the distal end also matches the pattern described.
It was also noted that the thinner portions of hair (ie near the root and at the tapering tip) lacked a medulla. That is to say, the widening of the mid region (which is the majority length of the fibre in each case) correlates with the presence of the medulla. This is particularly notable where the medulla appears near the root of the (five) guard hairs examined.
Writing in 1950, they also note that "only a few ... have made any detailed study of the surface structure of hair ... Hausman (1930) has shown that the cuticular scale types are directly related to the diameter of the hair shaft. ... Wynkoop (1929) has shown that age has little or no bearing on the microscopic appearance of the cuticular scales." (p 71).
The impression given in this study is that at the root end of guard hairs, where there is no medulla, the scale pattern appears imbricate. As the fibre widens (with the appearance of the medulla) a slightly spinuous appearance to the scales becomes apparent when examining the edges of the fibres as seen under the light microscope. It is possible that the growing medullary cells are causing the widening of the fibre, which in turn pushes the scales into a slightly spinuous position.
The under hair, in contrast, begins with a thinner root and the spinuous appearance of the scales is notable even before the medulla appears. If scales are of comparable absolute length regardless of hair type, then the thinner cortex of the under hair may explain why the scales are already spinuous even without the medulla being present; that is, the scales are "too large" to lay flat on such a thin fibre.
Lyne & McMahon state "Hausman (1930, p. 262) considers that the differences in scale form along a single hair "are the results of differences in the activities of the cells of the hair papilla, plus also, it is believed, some differences in the gradual drying out of the hair shaft, plus also the effects of wear and the free ectal edges of the scales, particularly at the tip of the shaft. The activities of the papillal cells produce differences both at the tip and the base of the shaft, i.e., when these cells begin, and close, their mitosis. Along the middle of the shaft the scales are fairly uniform in shape and relationships". The authors are of the opinion that neither dehydration nor attrition could cause the changes in scale form from the basal to the distal portions of a single hair. This is in close agreement with the findings of Wildman and Manby (1938, p. 343) in which they say that "A study of the scale-pattern along hairs, not only of the Monotremata but of other mammals, provides conclusive evidence that generally the changes in scale form from proximal to distal portions are innate and genetic in character, and are not caused by varying amounts of attrition".
This author finds the above conclusions generally representative of the Tasmanian tiger hairs examined in this study.
Atypical features observed
Three anomalous features were observed during this study. Firstly, the medullary cells took on a somewhat deflated appearance for short portions in the mid region of several guard hairs (see for example, file 3007). Secondly, the medulla of the over hair exhibited partial and/or irregular vacuolation, although this was in association with a break in the fibre (see for example, file 4040). In the same region of the break a longitudinal line was observed and it was concluded this appeared on the surface of the fibre facing the camera. It is not known whether these features existed in life, or whether they developed post mortem. Although the causes for these atypical features are not known, speculative causes are considered: the deflated medullary cells may have been due to nutritional deficiencies (explaining their appearance on multiple fibres) or decay post mortem. The line on the over hair may be a splitting of the fibre along one side, consistent with the break in the fibre. The vacuolation may have existed in life and weakened the fibre leading to the break, or the vauolation may suggest decay post-break.
Acknowledgements
The author would like to acknowledge the many backers of the light microscopy project, the project's sponsor, Dr Stephen Sleightholme for his support and guidance and Colin Veitch, CSIRO Manufacturing - Waurn Ponds Laboratory, for his assistance in interpretation of the micrographs and preparation of the scales.
Citing this article
Rehberg, C. (2017). OLM Optical Light micrographs of Tasmanian tiger hair, Accessed: date, http://www.wherelightmeetsdark.com.au.
References
- Deedrick and Koch (2004) Microscopy of Hair Part II: A Practical Guide and Manual for Animal Hairs. Forensic Science Communications July 2004 - Volume 6 - Number 3, Accessed: 3 November 2017, https://www.fbi.gov/
- Lyne, AG and McMahon, TS (1950) Observations on the surface structure of the hairs of Tasmanian monotremes and marsupials. Papers and Proceedings of the Royal Society of Tasmania. pp. 71-84. ISSN 0080-4703
- Taylor, Robert J (1985) Identification of the hair of Tasmanian mammals. Papers and Proceedings of the Royal Society of Tasmania 119, 1985. pp.69-82. 0080-4703
- Rehberg, C. (2017) SEM Scanning electron micrographs of Tasmanian tiger hair, Accessed: 9 June 2017, http://www.wherelightmeetsdark.com.au.
Rehberg, C. (2017) CLSM Confocal laser scanning 3D optical micrographs of Tasmanian tiger hair, Accessed: 10 October 2017, http://www.wherelightmeetsdark.com.au.
Rehberg, C. (2017) SEM Metal coated scanning electron micrographs of Tasmanian tiger hair, Accessed: 10 October 2017, http://www.wherelightmeetsdark.com.au.