SEM Metal coated scanning electron micrographs of Tasmanian tiger hair
Overview
A series of scanning electron micrographs of Tasmanian tiger hair (Thylacinus cynocephalus, also called "thylacine") was produced in 2017 by Rehberg (author), in collaboration with the Commonweath Scientific and Industrial Research Organisation (CSIRO).
Subsequent to the scanning electron microscopy (SEM) research, CSIRO retained hair fibres for the purpose of research using additional imaging techniques. A series of confocal laser scanning microscopy (CLSM) micrographs was also produced by CSIRO and published in 2017 by Rehberg.
CSIRO has continued its imaging of the thylacine hair fibres and a new set of SEM migrographs has been produced. For this sequence of imaging, the fibres have been coated in a metal alloy. The full set of metal-coated SEM micrographs is presented here.
Technique
The Tasmanian tiger's fur coat includes three distinct types of hair (Lyne & McMahon, 1950; Taylor, 1985). These are referred to as the over hair, guard hair and under hair (Taylor, 1985).
For the present study, one of each hair type was coated with ~3nm of platinum / palladium alloy. This coating provides electrical conduction yet cannot be seen by the electron beam during scanning electron microscopy. The coating is also non-oxidising which will preserve these fibres for any future research.
Imaging was carried out in an Hitachi S4300 SE/N Scanning Electron Microscope (SEM). The samples were imaged under high vacuum, at approximately 10-3 Pa pressure. This is in contrast to the earlier SEM work. Air, even at 50 Pa as in the earlier work, scatters the instrument's electron beam at low voltages resulting in loss of resolution. The higher pressure used in this work alleviates this effect, producing finer resolution images. Beam energy was 2 kV (in contrast to the earlier 20kV) which improves surface detail in the final images. The working distance was nominally 6 mm.
For further information on the SEM imaging process refer to Rehberg 2017.
Images
Below are the first published scanning electron micrographs of Tasmanian tiger hair utilising a metal alloy coating. For each image, two scans were performed and the second of each is presented here.
Note: images are labelled with a magnification (such as "Scanned at 1,000x"). Actual magnification will vary depending on your device display. Images also present a scale (such as "50um", meaning 50 micrometres) - above this measure is a series of 11 squares. The measure is the distance from the first to the last square. The space between two adjacent squares is one tenth of the measure (ie. if the label shows "50um" then the distance between adjacent squares is 5 micrometers).
Images can be uniquely referenced by the file name (eg. 'file ac').
All images are copyright.
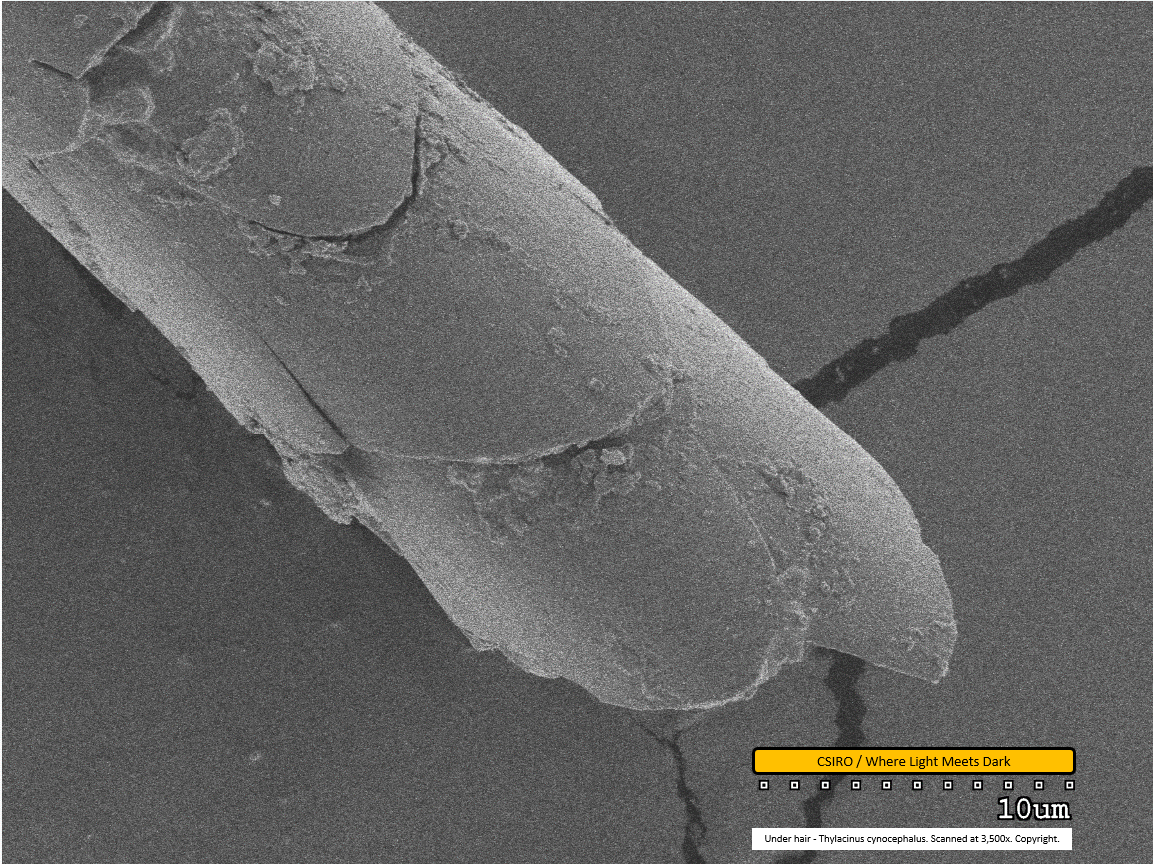
Under hair - distal end - scanned at 3,500x - file ac
This is the first of the under hair scans. The individual scales on the surface of the hair fibre are clearly visible in this image, with one seemingly split longitudinally. Dirt particles are adhered to the fibre.
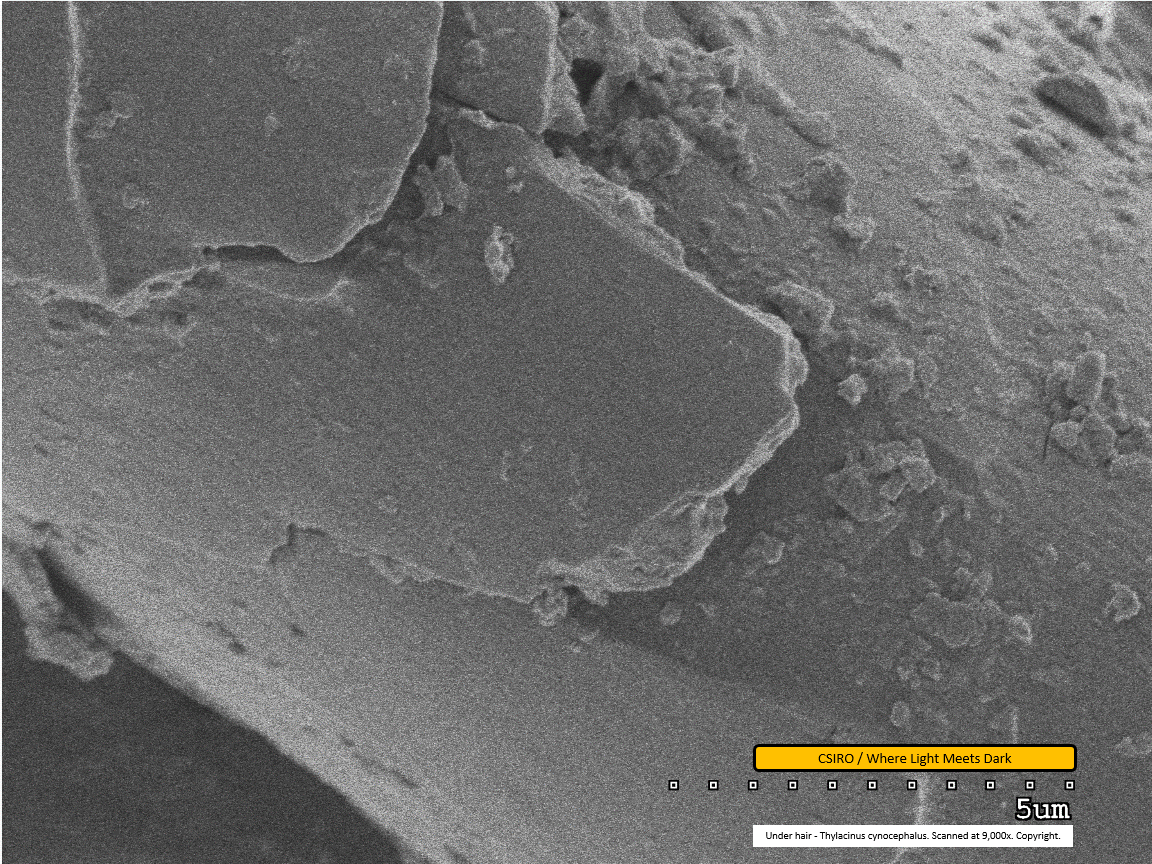
Under hair - mid region - scanned at 9,000x - file ad
This extremely close view of the surface of the hair fibre shows the jagged - possibly broken - edge of the fibre's surface scales. An impression of the thickness of the scale is visible along this edge. Dirt particles are adhered to the fibre.
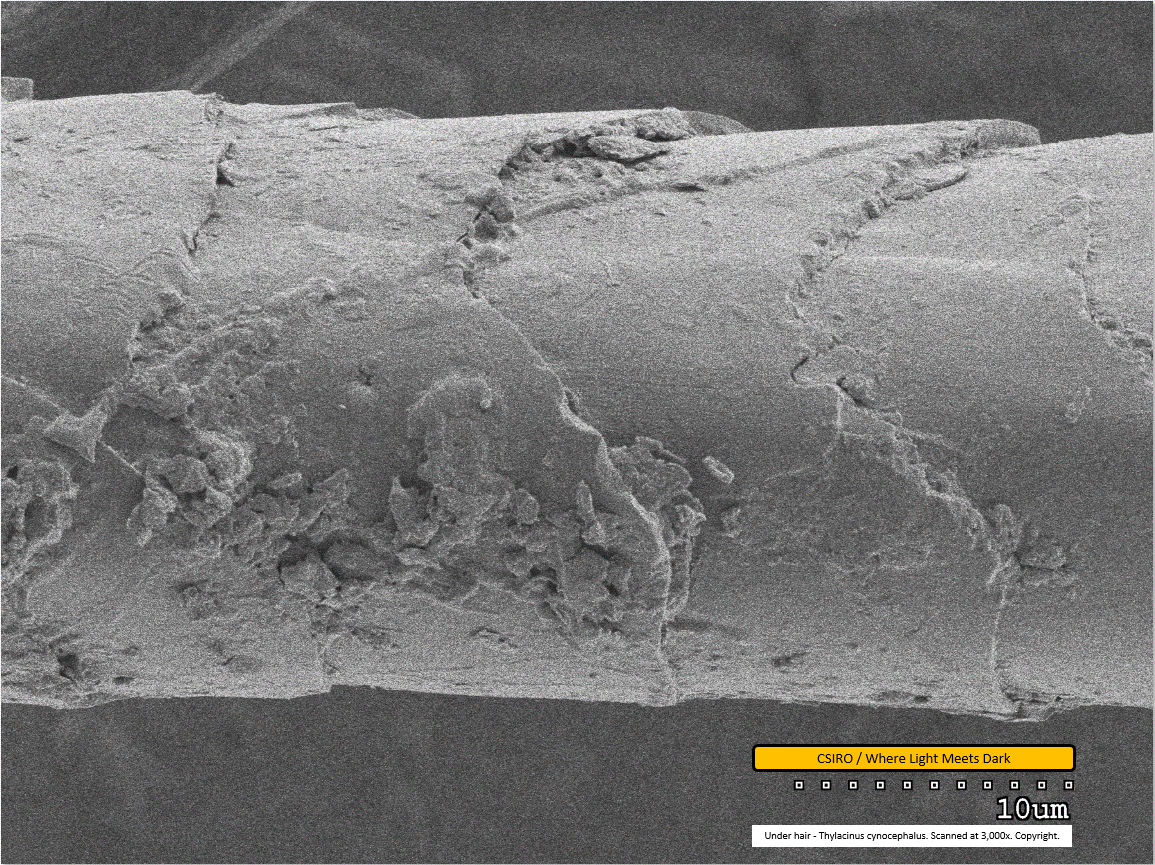
Under hair - mid region - scanned at 3,000x - file ae
Dirt particles are clearly visible adhered to the fibre in the lower half of this image and also where one scale overlaps the next at centre top of the image. It can be seen that scales overlap each other from left to right; that is, as you move down the fibre from left to right in this image, the left-most scale overlaps the subsequent one. This implies that the distal end of the fibre is toward the right.
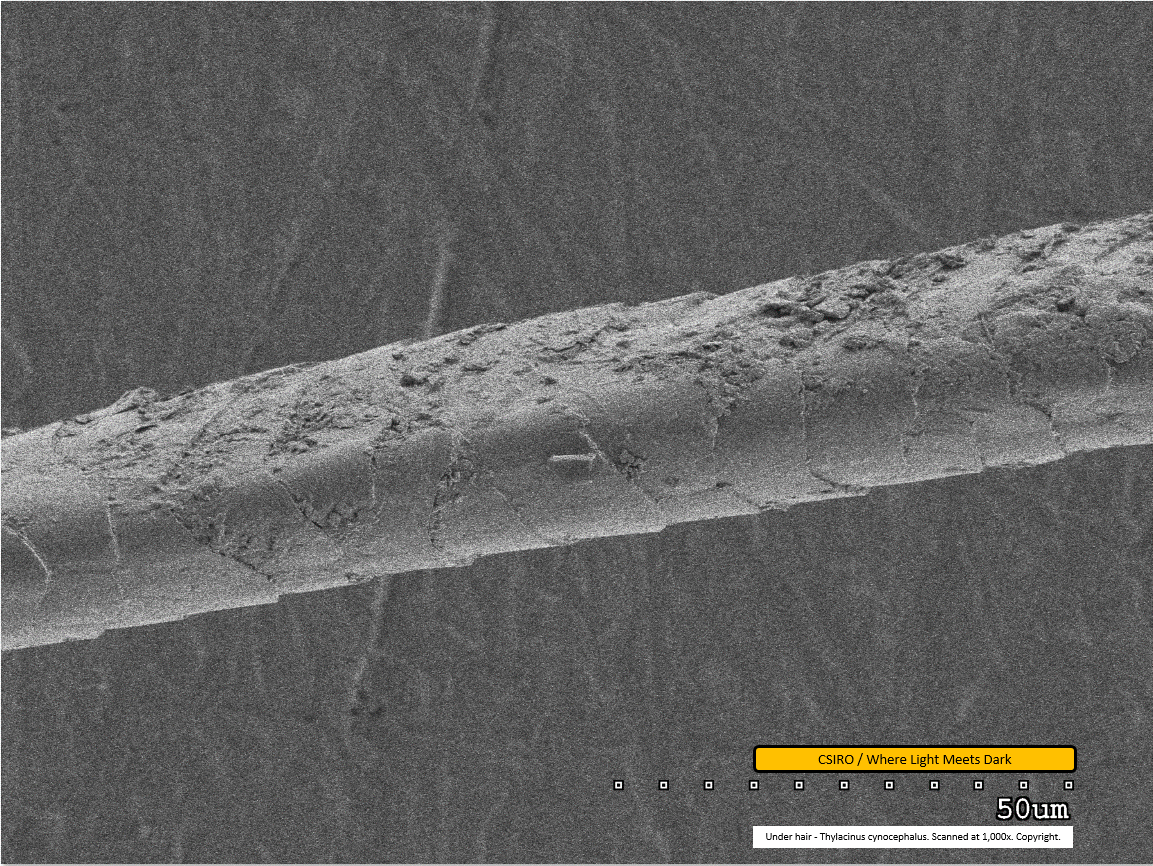
Under hair - mid region - scanned at 1,000x - file af
The scale pattern is clearly visible in this image, with the distal end of the fibre toward the right. The fibre appears to have been compressed at this point, possibly via the tweezers used for mounting. Dirt particles are visible adhered to the fibre.
Under hair - mid region - scanned at 500x - file ag
This scan at lower magnification gives a good impression of the scale pattern along the length of the fibre, visible as the pale bands traversing the fibre laterally. Dirt particles are clearly visible, especially at upper right in the image. The distal end of the fibre is toward the right.
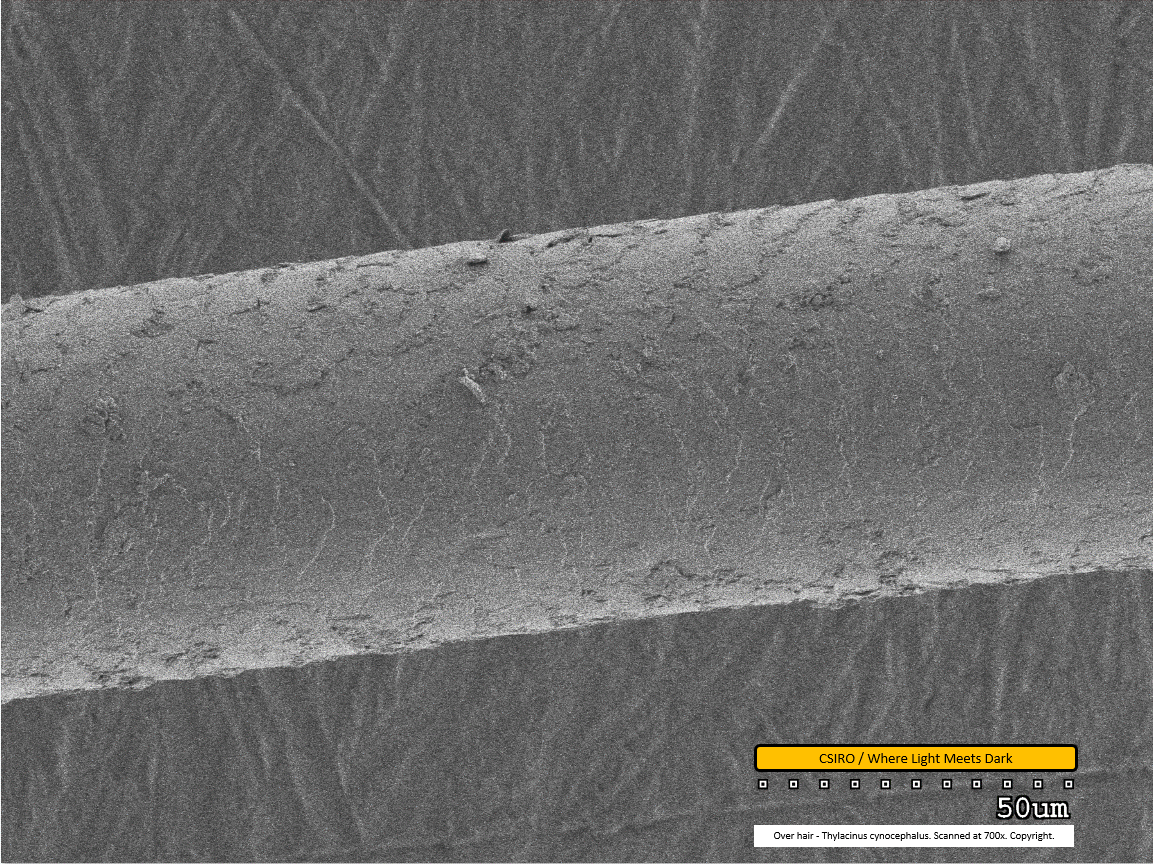
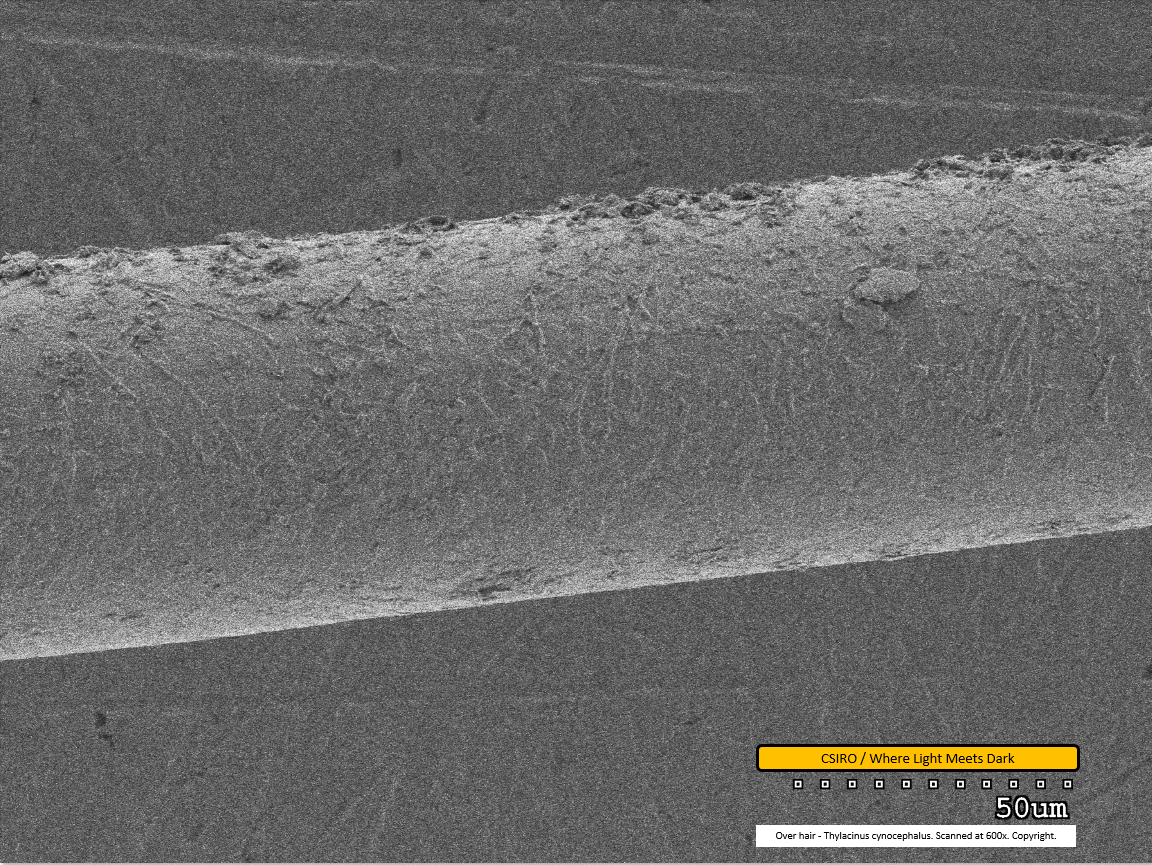
Over hair - mid region - scanned at 700x - file ah
This is the first of the over hair scans. The over hair is considerably thicker than the under hair. Its scale pattern is visible as both paler banding (along centre/bottom of fibre from this view) and darker banding (along top of fibre from this view). The scales overlap from left to right - that is, as you view the fibre from left to right within this image, each scale overlaps the subsequent one. This implies the distal end of the fibre is toward the right. Dirt particles are visible adhered to the fibre.
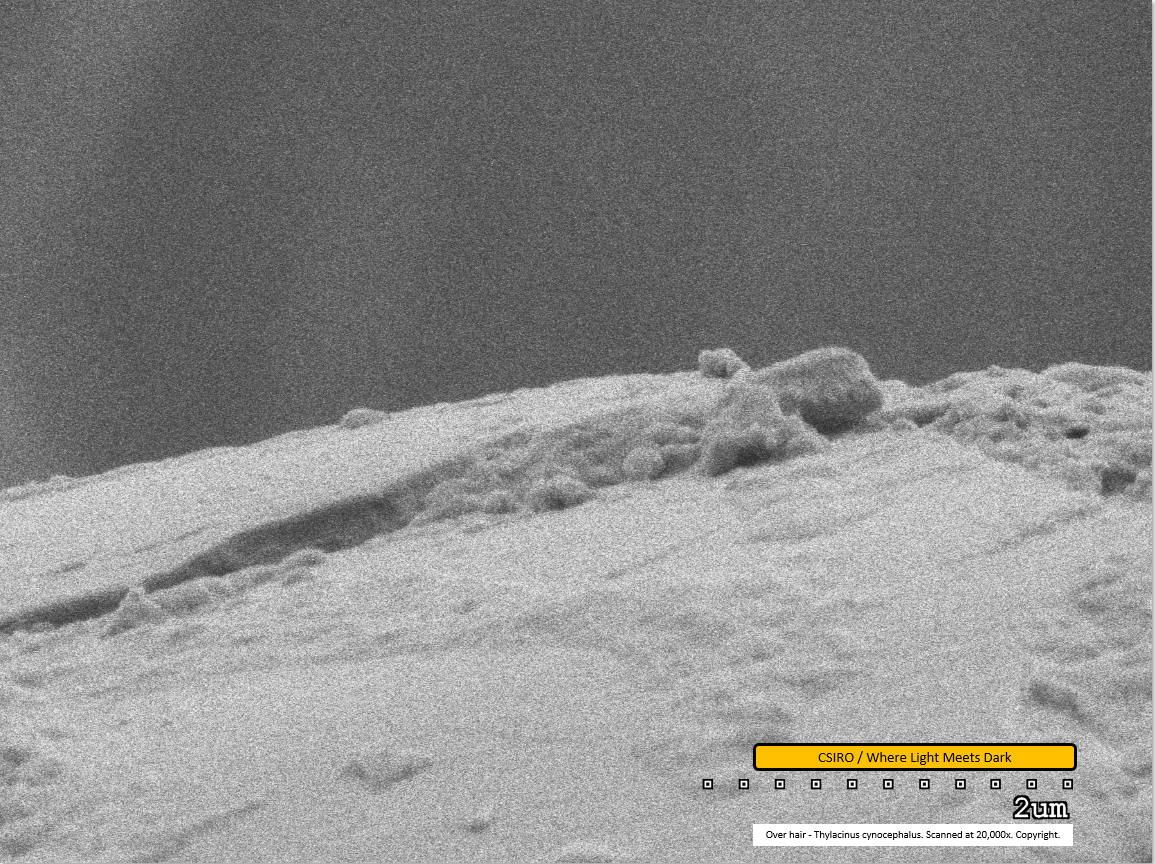
Over hair - mid region - scanned at 20,000x - file ai
This image presents the highest magnification scan performed on thylacine hair to date, at 20,000x magnification, along with file m in the original SEM work. It shows the junction of one scale overlapping another which produces the scale pattern visible at lower magnifications. In this image, the upper-left most edge of the fibre represents one scale and the ridge can be seen where the edge of the fibre drops down onto the next scale. A considerable amount of dirt is visible adhered to the fibre along much of this edge. The scale bar at lower right indicates a distance of 2 micrometers from end to end, suggesting the height of the ridge between scales is approx 0.4 micrometers.
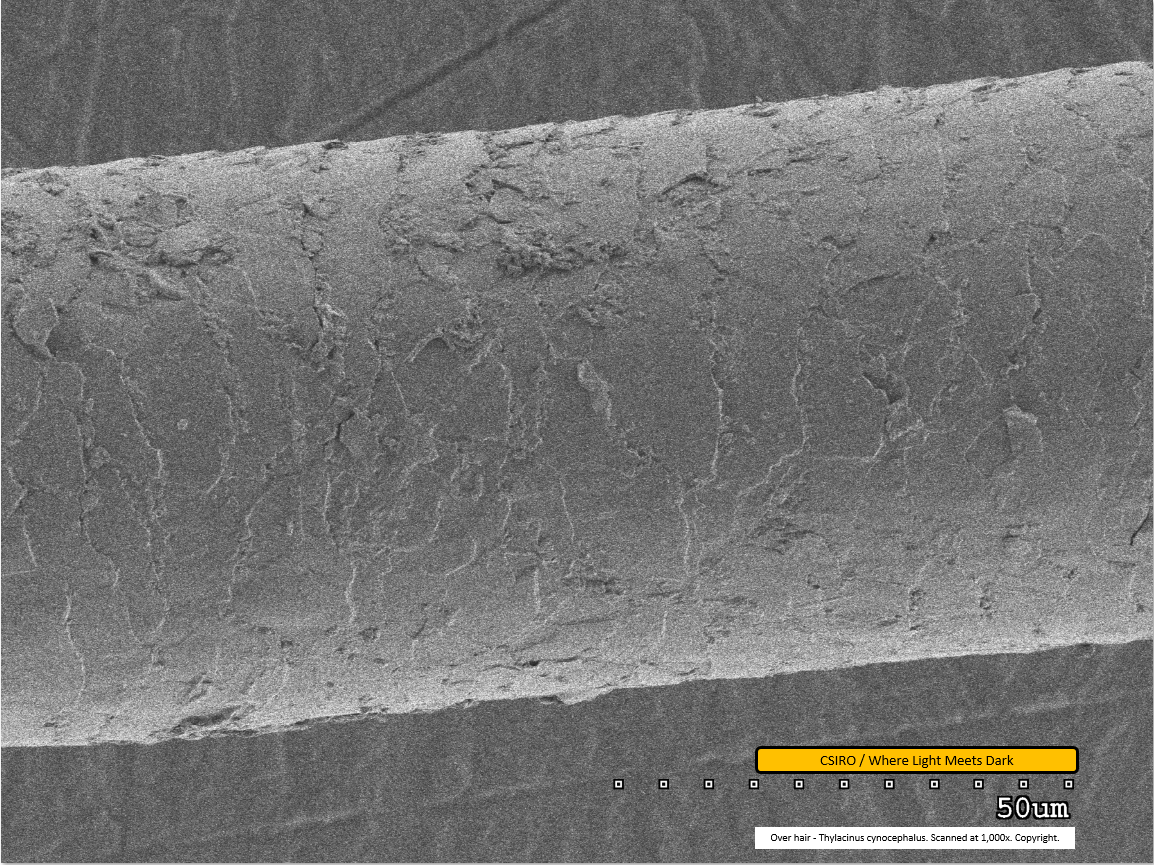
Over hair - mid region - scanned at 1,000x - file aj
This image of the over hair was scanned at 1,000x magnification. This is similar to file 'af', above, showing the under hair. Dirt particles are visible adhered to the fibre. The scale pattern can be clearly seen and is notably different from the pattern on the under hair.
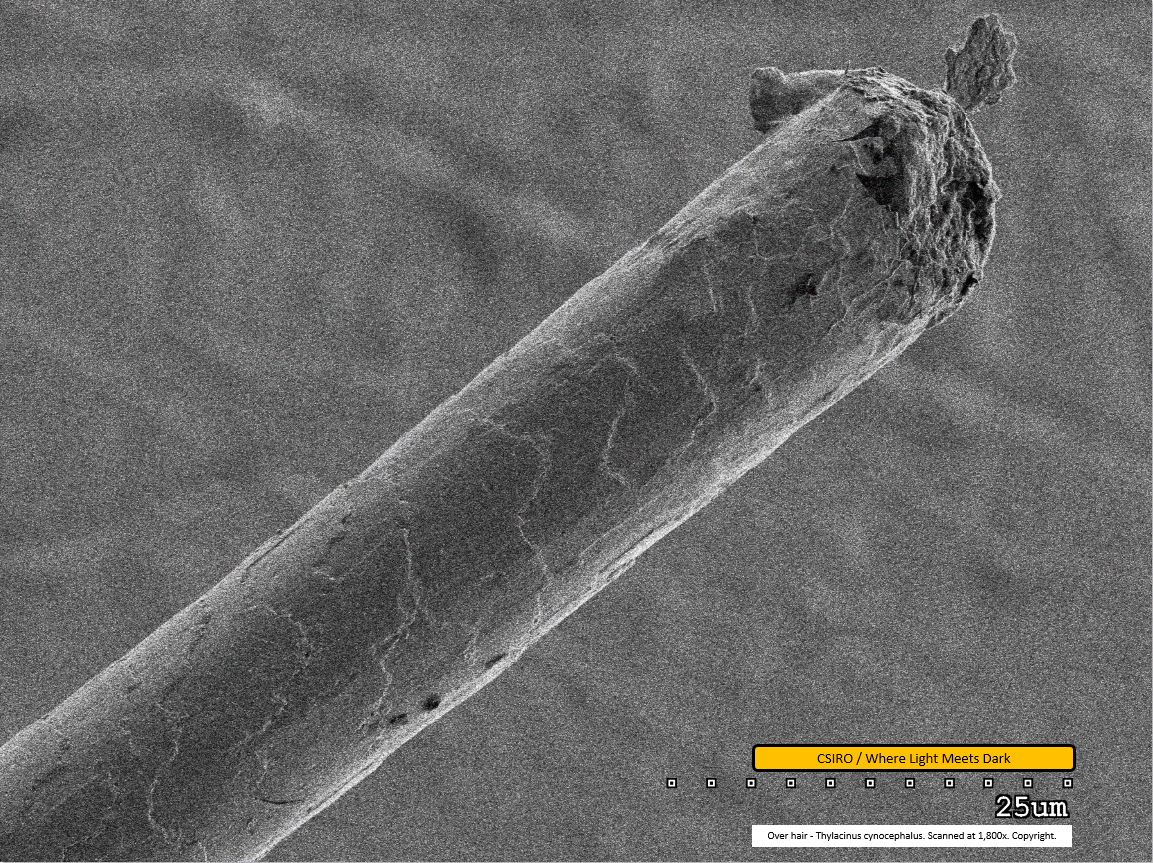
Over hair - distal end - scanned at 1,800x - file ak
This image shows the damaged distal end of the over hair. It is not clear what caused this damage; nor is it clear how close this break would have been to the fibre's natural end. Hair tissue - possibly a scale - is seen protruding unusually at the break point. Although this image was scanned at almost twice the magnification of the last one (file 'af'; 1,800x compared with 1,000x), the fibre appears only half as thick as it does in the prior scan. Hence it is clear the thickness of the over hair varies along its length. In correlation with this, the scale pattern is apparently different toward the distal end compared with the mid region scan. An observation is that scales essentially span similar lateral widths but are wrapped further around the smaller-diameter fibre than they are where the fibre is thicker. Accurate measurement may shed more light on how consistently this may be so.
Over hair - mid region - scanned at 600x - file al
This low magnification scan lacks some of the resolution visible in earlier images but the scale pattern is still partially discernible. Dirt particles are clearly adhered to the fibre.
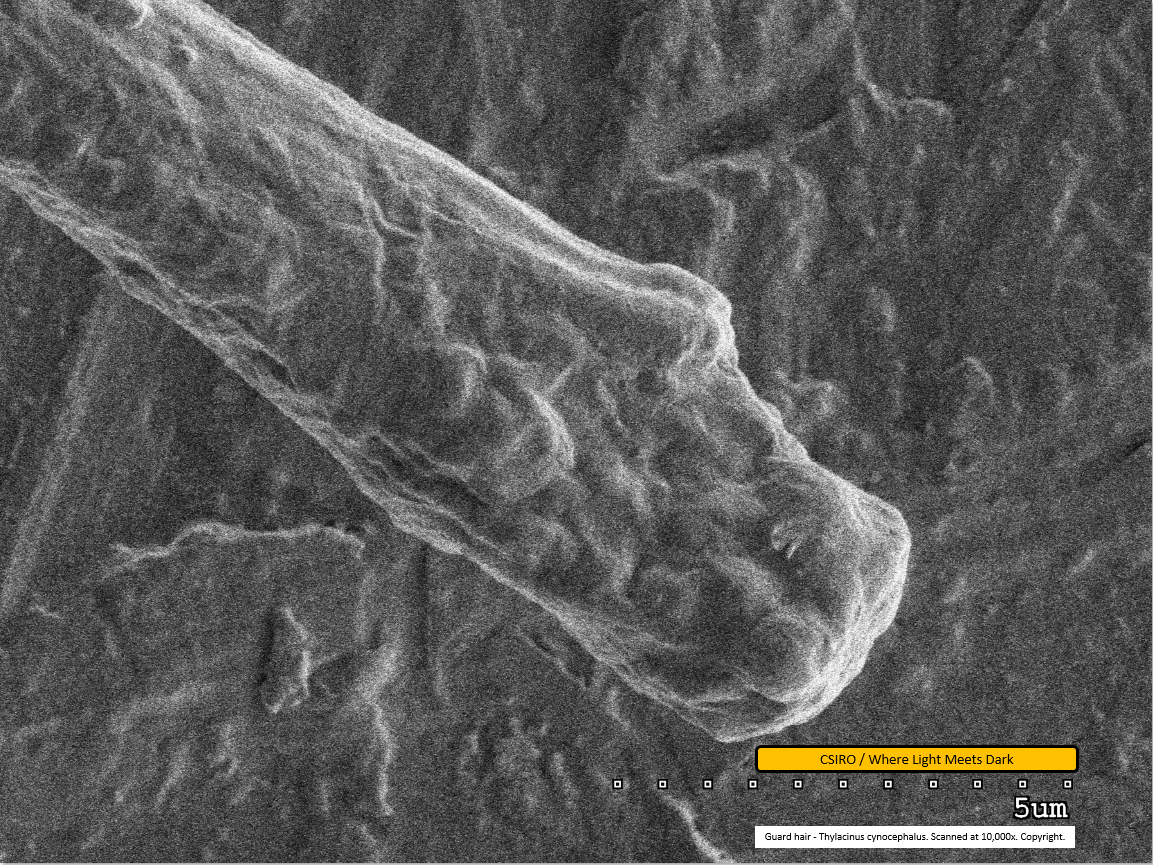
Guard hair - distal end - scanned at 10,000x - file am
This is the first of the guard hair scans. It shows the distal end at a magnification of 10,000x. This scan lacks the sharpness of other images in this series and it is difficult to discern detailed features.
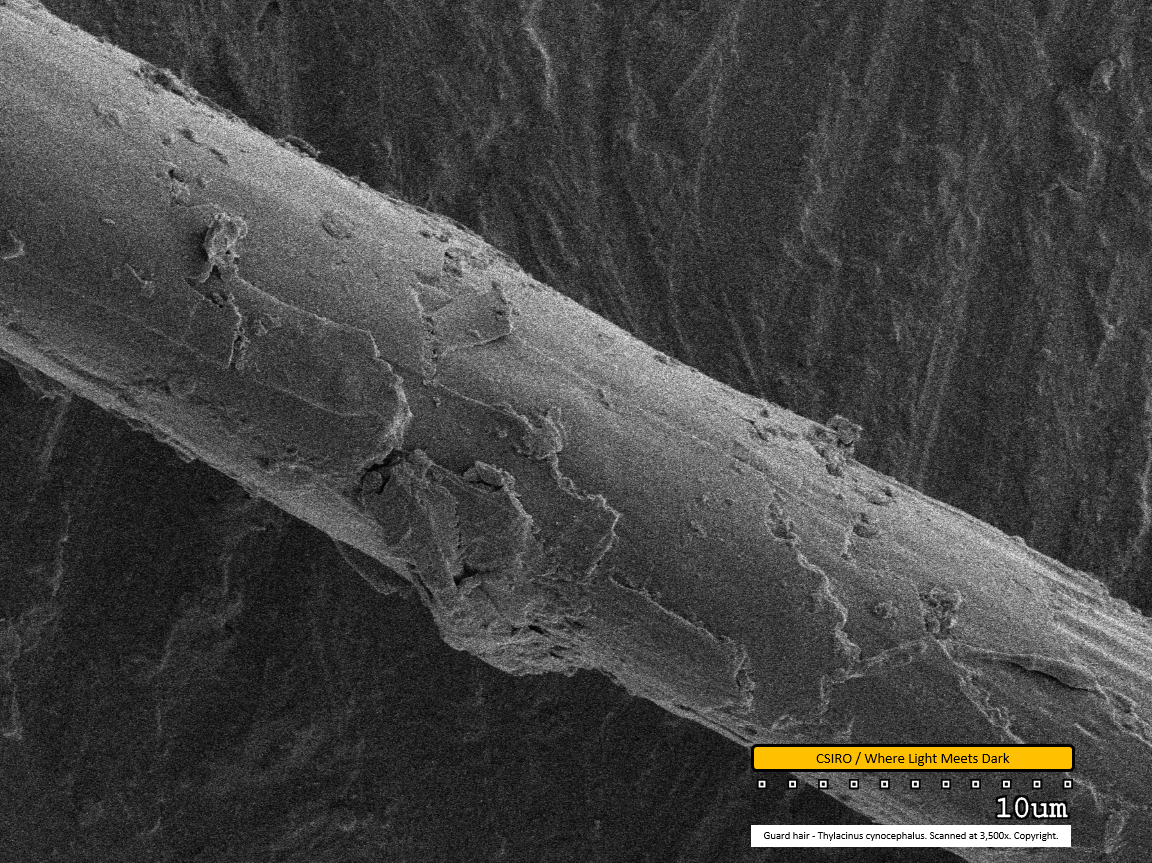
Guard hair - mid region - scanned at 3,500x - file an
This image clearly shows the scale edges. Scales toward the left of this image overlap the adjacent scales toward the right, indicating the distal end of the fibre is toward the right. Dirt particles are seen adhered to the fibre, especially at centre of the lower edge. Above this, a portion of the scale can be seen broken away and to the left of the broken scale, another scale is curling up and away from the fibre.
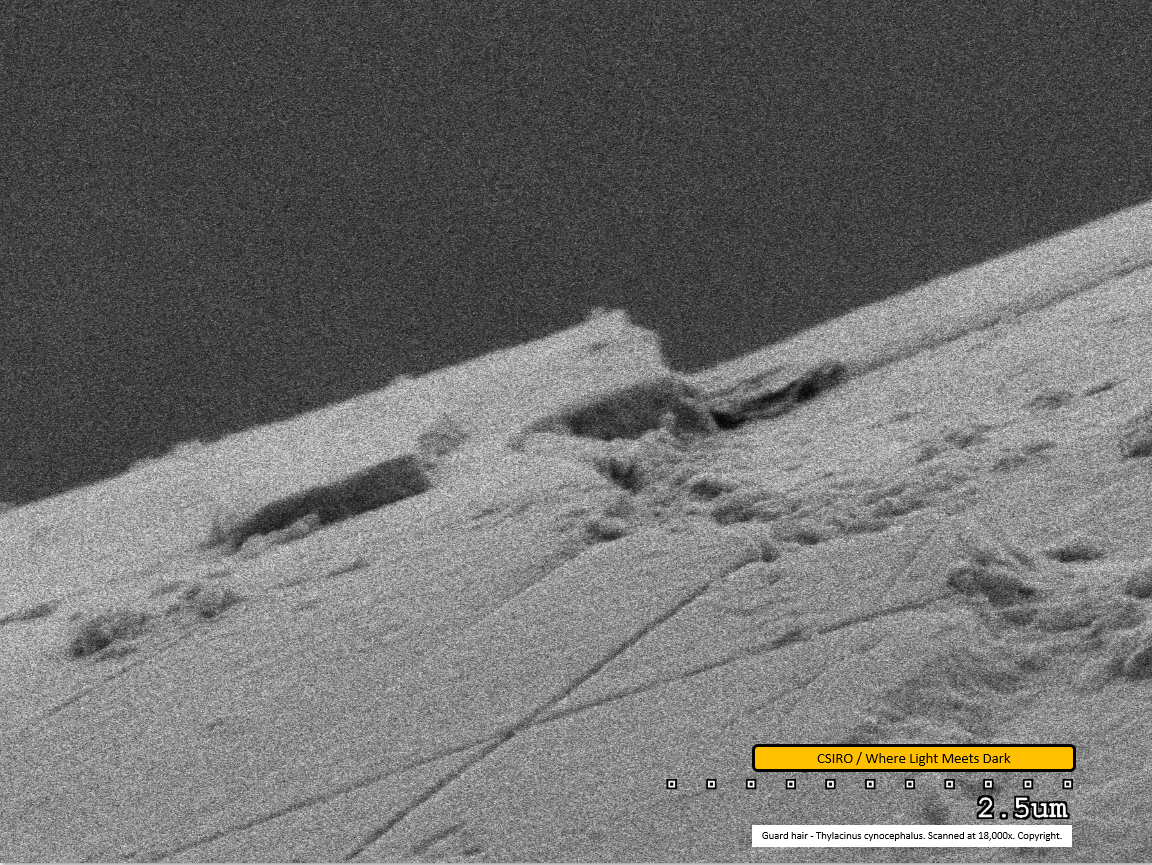
Guard hair - mid region - scanned at 18,000x - file ao
This scan, at very high magnification, shows one scale overlapping the next. Some dirt is visible adhered to the fibre. In the lower right portion of the scan, a build-up of material is seen in the groove between scales. The material has a curved appearance and is likely to constitute natural oils, similar to that seen in the earlier SEM work. The lower centre portion of the image appears to show two very straight, shallow grooves in the scale, forming an 'X' shape. It is not clear what has caused this appearance.
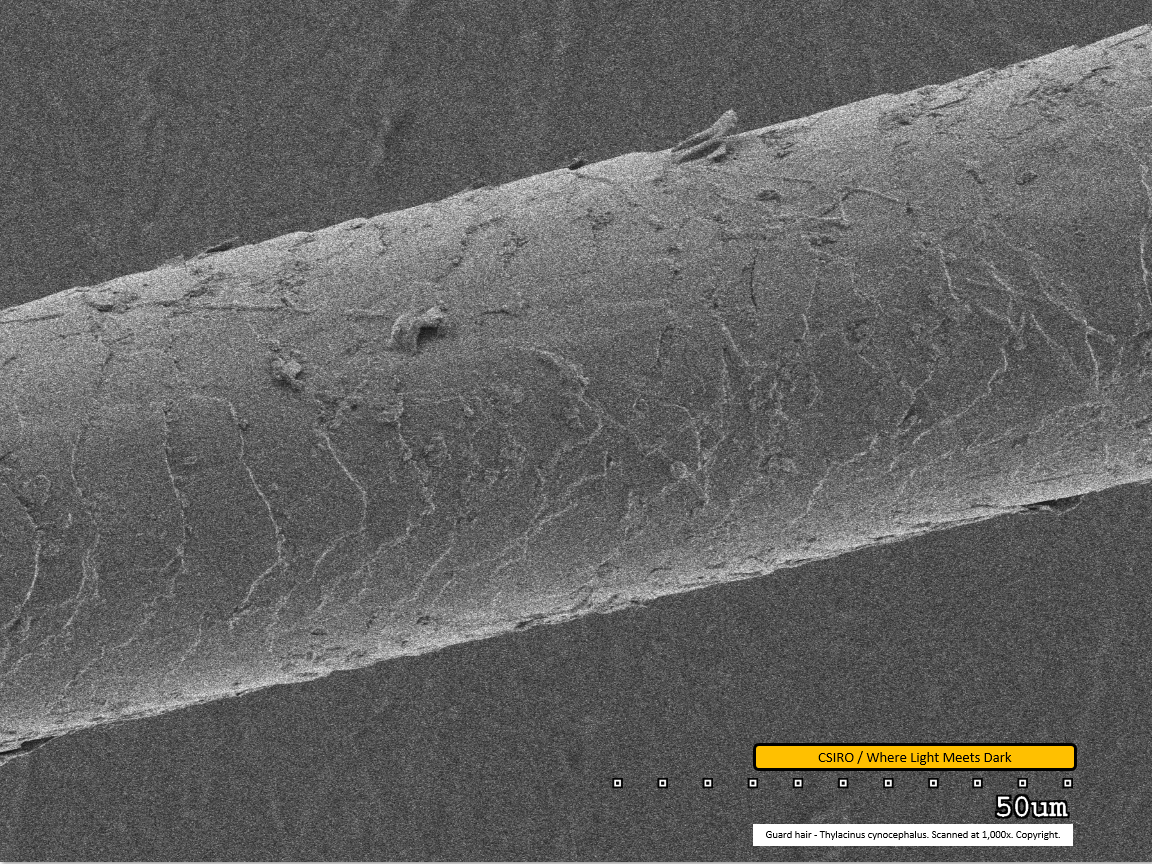
Guard hair - mid region - scanned at 1,000x - file ap
The scale pattern is clearly visible as a series of paler and darker transverse lines across the hair fibre. A few large dirt particles are visible adhered to the fibre.
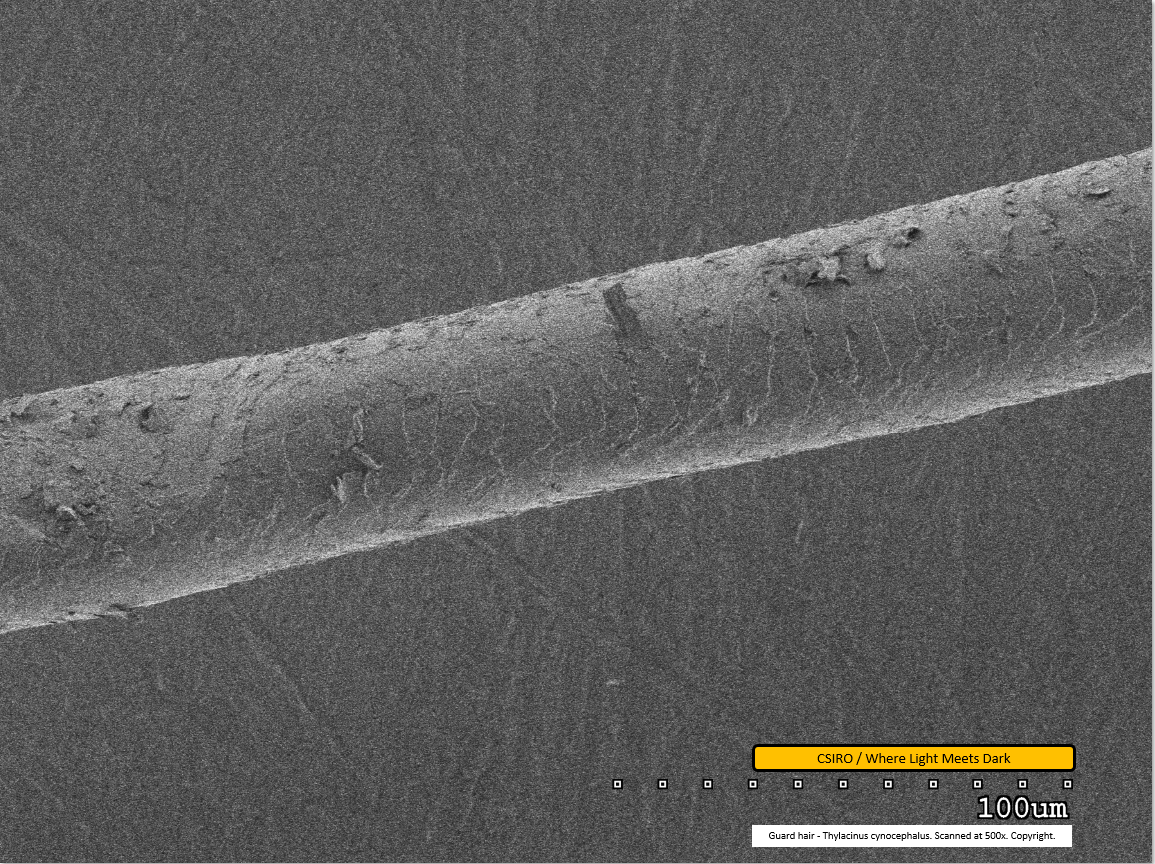
Guard hair - mid region - scanned at 500x - file aq
The scale pattern is visible as a series of paler lines traversing the fibre. At the left edge of the image the fibre appears to have been compressed - possibly by tweezers during preparation. Dirt particles are visible adhered to the fibre.
Further research and acknowledgements
This scanning work was carried out by CSIRO under a generous offer of support in response to ABC News coverage of Where Light Meets Dark's crowd funding campaign to produce high quality light microscopy images of Tasmanian tiger hair. The author would like to acknowledge the generosity of Colin Veitch, CSIRO Manufacturing - Waurn Ponds Laboratory and the coverage of ABC.
The stated goal of the crowd funding project is to produce light microscopy images of Tasmanian tiger hair that may become a reference base against which researchers may compare samples collected in the field for the purpose of a potential thylacine match. Although the scanning electron microscopy has produced unprecedented detail and magnification for thylacine hair, it is limited to surface morphology only. Light microscopy can reveal internal features of hair fibres, including the medulla (core) and this is also diagnostic in identifying unknown hair fibres. Further, SEM resources are expensive and not necessarily accessible to most researchers (particularly field researchers in search of the thylacine). Light microscopy is affordable at a much more local scale and so there is still a need to complete the light microscopy work before a useful catalogue of images is available for comparative analysis.
The author would also like to acknowledge the many backers of the light microscopy project, and the project's sponsor, Dr Stephen Sleightholme, without whom there would not have been news coverage and the generous offer from CSIRO for SEM.
Citing this article
Rehberg, C. (2017). SEM Metal coated scanning electron micrographs of Tasmanian tiger hair, Accessed: date, http://www.wherelightmeetsdark.com.au.
References
- Lyne, AG and McMahon, TS (1950) Observations on the surface structure of the hairs of Tasmanian monotremes and marsupials. Papers and Proceedings of the Royal Society of Tasmania. pp. 71-84. ISSN 0080-4703
- Taylor, Robert J (1985) Identification of the hair of Tasmanian mammals. Papers and Proceedings of the Royal Society of Tasmania 119, 1985. pp.69-82. 0080-4703
- Rehberg, C. (2017) SEM Scanning electron micrographs of Tasmanian tiger hair, Accessed: 9 June 2017, http://www.wherelightmeetsdark.com.au.
Rehberg, C. (2017) CLSM Confocal laser scanning 3D optical micrographs of Tasmanian tiger hair, Accessed: date, http://www.wherelightmeetsdark.com.au.